|
There
is usually confusion between electrolytic and galvanic corrosion. The difference is simple, while galvanic corrosion is caused by an
electric current generated by two different materials in a conductive medium such as salt water, electrolytic corrosion is caused by a current produced by an external source, usually
the battery of the boat or some other electrical source.
In a technical sense, the term electrolysis refers to the decomposition of a chemical produced by the flow of electric current through it. This means that even two similar materials can
be converted into both the cathode and the anode of a cell, in which the anode would be the corroded.
|
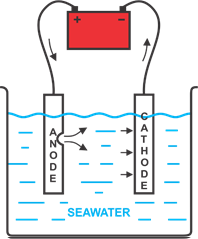
But to better comprehend the general term of electrolytic corrosion, we must first understand 4 terms separately: CORROSION,
ELECTROLISIS, CATHODE AND ANODE.
CORROSION is the wear or deterioration of a piece of any material by electrochemical action, which applied to metals can normally be seen as oxidation, where the oxygen in the environment
is responsible for breaking down the molecular structure of metals making them fragile and giving them that reddish color characteristic of metals such as iron and steel or a dark green
finish by patina to copper and its alloys.

|
|
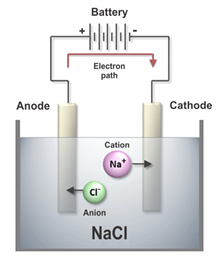
ELECTROLISIS is the means by which a chemical substance is decomposed by the effect of an electric current under an aqueous solution, where two important factors act in this process,
which are the electrodes: the CATHODE, which is connected to the negative pole of the electric current and the ANODE, which goes to the positive pole.
Electrolysis shows the molecular decomposition of the cathode attracting the anode to the negative ions (anions) of the cathode and the cathode attracting the positive ions of the anode
(cations). In this oxide-reduction reaction, the anode is oxidized by attracting oxygen separated from hydrogen in the aqueous solution while the cathode is reduced.
In salt water, electrolysis not only breaks down the water molecules, but all the minerals found in the solution, causing all positive salt water ions to be received by the anode.
|
|
In the hull of a ship, the electric current produced by electrolysis is caused by errors in the boat's electrical system and the conductivity of the material from which the hull
itself is made. The main causes of electrolytic corrosion are caused by current leaks that find electric grounding in the hull, for example, a bad electrical installation, a short
circuit or a badly grounded of some tool or electronic equipment such as communication systems, for example, or by current spills under humid conditions. A bad wiring in an aluminum
hull, which is highly conductive, can cause the electrolysis process and cause parts such as the propeller and the nozzle to decompose due to this phenomenon. The only solution for this
problem is a good electrical installation without leaks, besides taking into account never using the hull as a ground in the electrical system.
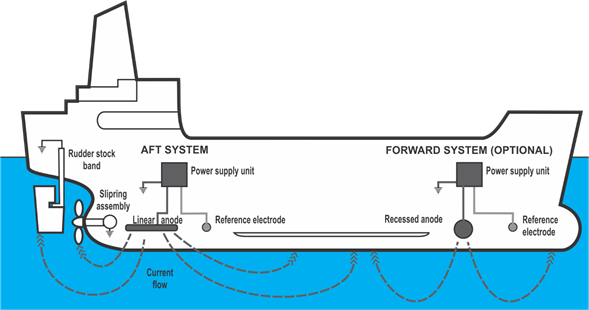
The wiring system should always be isolated in the return circuit (two wires) instead of grounded. A master switch must be connected to the positive battery terminal and turned off when
the vessel is at rest. Grounding is required for safety when there are high voltages, for example, with the installation of a 240-volt generator or the installation of a supply strut. In
this case the ground must not be confused with the return ground. The return ground always carries current while the ground involves a third cable that does not carry current. This
grounding is carried out by connecting an insulated cable to a sacrificial plate placed at the bottom of the hull, as far as possible from the propellers.
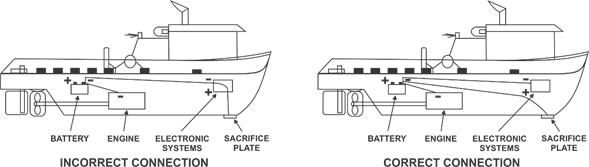
Examples of an incorrect and a correct grounding:
|